


You are leaving PADCEV.com
By clickingtapping "Go to link" below, you are leaving an Astellas, Inc. and Seattle Genetics, Inc. website. Astellas, Inc. and Seattle Genetics, Inc. are not responsible for the information or services on any third-party sites.
This link is provided to you only as a convenience, and the inclusion of any link does not imply endorsement by Astellas Inc. and/or Seattle Genetics, Inc. of the site, its contents, or the owner of the site. Please review the privacy policy and terms of use of the site to understand how your information will be processed.
Thank you for visiting our site.


Back to Top
What is PADCEV™ and how does it work?
PADCEV works differently from the chemotherapy or immunotherapy you may have had before because PADCEV is an antibody-drug conjugate (ADC). PADCEV works by delivering medicine directly to cancer cells, but can also harm normal cells and cause side effects. Talk to your doctor about side effects and click here for information about side effects with PADCEV.


PADCEV works differently from the chemotherapy or immunotherapy you may have had before because PADCEV is an antibody-drug conjugate (ADC). PADCEV works by delivering medicine directly to cancer cells, but can also harm normal cells and cause side effects. Talk to your doctor about side effects and click here for information about side effects with PADCEV.
PADCEV is made of 3 parts:





How PADCEV is thought to work:
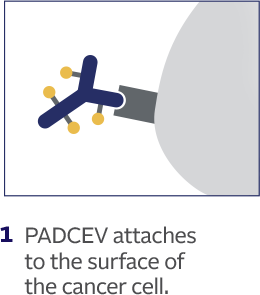
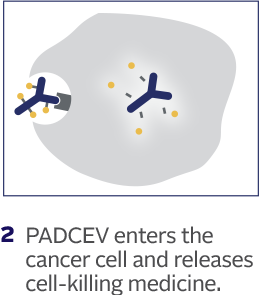
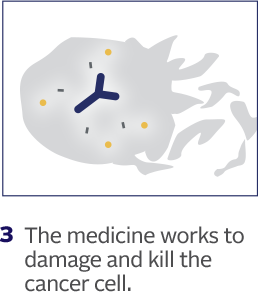
This is how PADCEV was shown to work in laboratory studies.
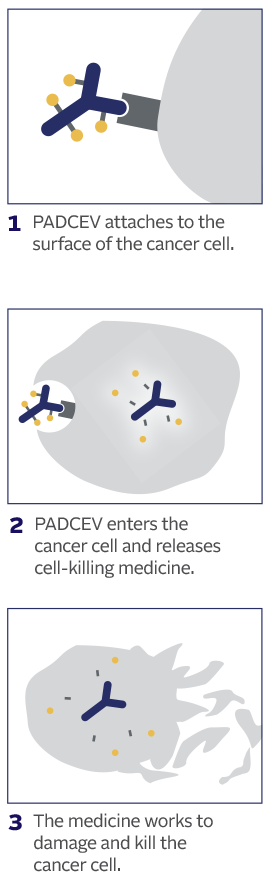
This is how PADCEV was shown to work in laboratory studies.

Antibody: A protein most commonly made by the body’s immune system. The antibody that makes up PADCEV is made in a laboratory
How was PADCEV shown to help?
PADCEV was studied in a clinical trial of 125 adults with advanced bladder cancer who had received
an immunotherapy medicine and
chemotherapy-containing platinum medicine before treatment with PADCEV.*


How was PADCEV shown to help?
PADCEV was studied in a clinical trial of 125 adults with advanced bladder cancer who had received
an immunotherapy medicine and
chemotherapy-containing platinum medicine before treatment with PADCEV.*
PADCEV was studied in a clinical trial of 125 adults with advanced bladder cancer who had received
an immunotherapy medicine and
chemotherapy-containing platinum medicine before treatment with PADCEV.*
In the clinical trial of PADCEV:



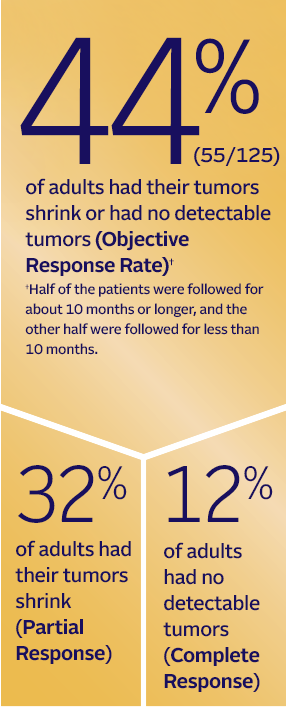
*PADCEV's approval was based on a study of 125 adults who had previously been treated with immunotherapy medicine and chemotherapy-containing platinum medicine.

Chemotherapy: Treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. This can affect cancer cells and normal cells
Immunotherapy: Treatment that uses substances to stimulate or suppress the immune system to help the body fight cancer, infection, and other diseases
Objective response rate: A measurable response to a treatment that includes partial response and complete response
Partial response: When the tumors partly responded to treatment, but still did not go away. In the PADCEV trial, a decrease in the size of a tumor by 30% was considered a partial response
Complete response: When a treatment completely gets rid of all tumors that could be measured or seen on a test
What are some of the possible side effects of PADCEV?
PADCEV may cause serious side effects, including: high blood sugar (hyperglycemia), peripheral neuropathy, eye problems, skin reactions, and leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). Click here for more information on serious side effects.


What are some of the possible side effects of PADCEV?
PADCEV may cause serious side effects, including: high blood sugar (hyperglycemia), peripheral neuropathy, eye problems, skin reactions, and leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). Click here for more information on serious side effects.
PADCEV may cause serious side effects, including: high blood sugar (hyperglycemia), peripheral neuropathy, eye problems, skin reactions, and leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation).
Click here for more information on serious side effects.
The following are the most common side effects of PADCEV:

Numbness or tingling in your hands or
feet, or muscle weakness

Nausea

Fatigue

Diarrhea

Decreased appetite

Change in sense of taste

Rash

Dry eyes

Hair loss

Dry skin

Nausea

Diarrhea

Change in sense of taste

Dry eyes

Dry skin
PADCEV may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare
provider if you have concerns about fertility.
You and your caregiver(s) should always talk to your doctor or nurse right away about any side effects you may experience, or if these get worse.


IMPORTANT SAFETY INFORMATION

Before receiving PADCEV, tell your healthcare provider about all of your medical conditions, including if you:
• are currently experiencing numbness or tingling in your hands or feet
• have a history of high blood sugar or diabetes
• are pregnant or plan to become pregnant. PADCEV can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with PADCEV
• are breastfeeding or plan to breastfeed. It is not known if PADCEV passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of PADCEV

Females who are able to become
pregnant:
• Your healthcare provider should do a pregnancy test before you start treatment with PADCEV.
• You should use an effective method of birth control during your treatment and for at least 2 months after the last dose of PADCEV.

Males with a female sexual partner
who is able to become pregnant:
• If your female partner is pregnant, PADCEV can harm the unborn baby.
• You should use an effective method of birth control during your treatment and for at least 4 months after the last dose of PADCEV.

Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.

How will you receive PADCEV?
• PADCEV will be given to you by intravenous (IV) infusion into your vein over 30 minutes.
• You will receive your PADCEV over periods of time called cycles.
- Each PADCEV cycle is 28 days.
- You will receive PADCEV on days 1, 8 and 15 of every cycle.
• Your healthcare provider will decide how many treatment cycles you need.
• Your healthcare provider may do blood tests regularly during treatment with PADCEV.
What are the possible side effects of PADCEV?
PADCEV may cause serious side effects including:

• High Blood Sugar (hyperglycemia). You can develop high blood sugar during treatment with PADCEV. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including: frequent urination, increased thirst, blurred vision, confusion, it becomes harder to control your blood sugar, drowsiness, loss of appetite, fruity smell on your breath, nausea, vomiting, or stomach pain.

• Peripheral neuropathy. While receiving PADCEV you may experience nerve problems called peripheral neuropathy. Tell your healthcare provider right away if you experience numbness or tingling in your hands or feet, or muscle weakness.

• Eye problems. You can develop certain eye problems while receiving PADCEV. Tell your healthcare provider right away if you have dry eyes or blurred vision.

• Skin reactions. Rashes and severe skin reactions can happen while receiving PADCEV. Tell your healthcare provider right away if you get a rash or a skin reaction that continues to get worse.

• Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, or discomfort at the infusion site.
The most common side effects of PADCEV
include:
• numbness or tingling in your hands or feet, or muscle weakness
• fatigue
• decreased appetite
• rash
• hair loss
• nausea
• diarrhea
• change in sense of taste
• dry eyes
• dry skin
If you have certain side effects, your healthcare provider may decrease your dose or stop your treatment with PADCEV for a period of time (temporarily) or completely.
PADCEV may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.

These are not all the possible side effects of PADCEV.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please click here for full Prescribing Information.
WHAT IS PADCEV™?
PADCEV (enfortumab vedotin-ejfv) is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery. PADCEV may be used if you have received an immunotherapy medicine and also received a chemotherapy-containing platinum medicine. It is not known if PADCEV is safe and effective in children.
PADCEV was FDA-approved based on a clinical study that measured how many patients had a tumor response. There is another study with PADCEV to confirm the clinical benefit.
Please click here for full Prescribing Information.


IMPORTANT SAFETY INFORMATION

Before receiving PADCEV, tell your healthcare provider about all of your medical conditions, including if you:
• are currently experiencing numbness or tingling in your hands or feet
• have a history of high blood sugar or diabetes
• are pregnant or plan to become pregnant. PADCEV can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with PADCEV
• are breastfeeding or plan to breastfeed. It is not known if PADCEV passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of PADCEV

Females who are able to become
pregnant:
• Your healthcare provider should do a pregnancy test before you start treatment with PADCEV.
• You should use an effective method of birth control during your treatment and for at least 2 months after the last dose of PADCEV.

Males with a female sexual partner
who is able to become pregnant:
• If your female partner is pregnant, PADCEV can harm the unborn baby.
• You should use an effective method of birth control during your treatment and for at least 4 months after the last dose of PADCEV.

Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.

How will you receive PADCEV?
• PADCEV will be given to you by intravenous (IV) infusion into your vein over 30 minutes.
• You will receive your PADCEV over periods of time called cycles.
- Each PADCEV cycle is 28 days.
- You will receive PADCEV on days 1, 8 and 15 of every cycle.
• Your healthcare provider will decide how many treatment cycles you need.
• Your healthcare provider may do blood tests regularly during treatment with PADCEV.
What are the possible side effects of PADCEV?
PADCEV may cause serious side effects including:

• High Blood Sugar (hyperglycemia). You can develop high blood sugar during treatment with PADCEV. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including: frequent urination, increased thirst, blurred vision, confusion, it becomes harder to control your blood sugar, drowsiness, loss of appetite, fruity smell on your breath, nausea, vomiting, or stomach pain.

• Peripheral neuropathy. While receiving PADCEV you may experience nerve problems called peripheral neuropathy. Tell your healthcare provider right away if you experience numbness or tingling in your hands or feet, or muscle weakness.

• Eye problems. You can develop certain eye problems while receiving PADCEV. Tell your healthcare provider right away if you have dry eyes or blurred vision.

• Skin reactions. Rashes and severe skin reactions can happen while receiving PADCEV. Tell your healthcare provider right away if you get a rash or a skin reaction that continues to get worse.

• Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, or discomfort at the infusion site.
The most common side effects of PADCEV
include:
• numbness or tingling in your hands or feet, or muscle weakness
• fatigue
• decreased appetite
• rash
• hair loss
• nausea
• diarrhea
• change in sense of taste
• dry eyes
• dry skin
If you have certain side effects, your healthcare provider may decrease your dose or stop your treatment with PADCEV for a period of time (temporarily) or completely.
PADCEV may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.

These are not all the possible side effects of PADCEV.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please click here for full Prescribing Information.
WHAT IS PADCEV™?
PADCEV (enfortumab vedotin-ejfv) is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery. PADCEV may be used if you have received an immunotherapy medicine and also received a chemotherapy-containing platinum medicine. It is not known if PADCEV is safe and effective in children.
PADCEV was FDA-approved based on a clinical study that measured how many patients had a tumor response. There is another study with PADCEV to confirm the clinical benefit.
Please click here for full Prescribing Information.
